MICROBIOLOGY RESEARCH
Biofilms of Bordetella avium and Bordetella hinzii
Table of Contents
What I loved about my undergraduate research
Compared to some of the more “popular” Bordetella species, B. avium and B. hinzii have little published research. When I first started in this lab, this fact made me nervous. How do I figure out what and how to investigate when there isn’t a massive body of research already? How will I know what methods work best with these bacteria? Science experiments can be so finicky and finding your footing in unknown waters can be intimidating. To my surprise, this was a considerable advantage in developing my skills in experimental design, investigation (asking the right questions), and problem-solving. While a lot of research can be monotonous and painful (hand cramps from pipetting for 20 minutes, anyone?), I was never bored in the beginning stages of planning and strategizing.
I will be forever grateful for my time in this research lab. Dr. Louise Temple was a phenomenal mentor who (still) inspires me (and always will).
What are Bordetella avium and Bordetella hinzii?
Bordetella avium and Bordetella hinzii (or B. avium and B. hinzii for short) are two bacteria species that infect turkeys. They cause respiratory infections referred to as bordetellosis. In turkeys, this infection is fatal to young or weak birds and causes a characteristic cough called “snicking”.
For a while, it was believed that only B. avium caused sickness in turkeys, but it was later revealed that B. hinzii was also involved in these infections. With this, I had one main question that I wanted to explore:
How do these two bacteria species interact with each other?
You may be familiar with B. avium and B. hinzii’s cousins, B. pertussis and B. bronchiseptica. B. pertussis is the culprit responsible for whooping cough in humans and B. bronchiseptica is responsible for kennel cough in dogs.
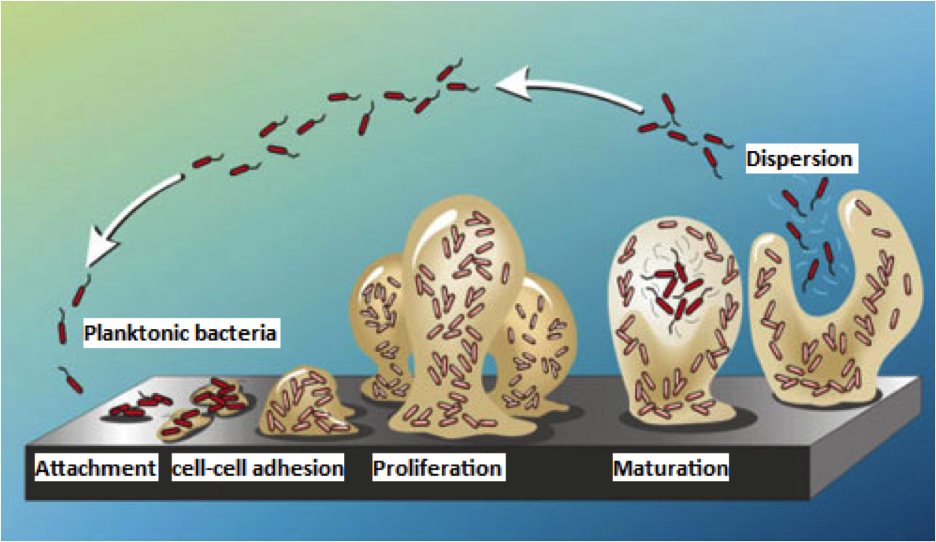
What are biofilms?
Biofilms are essentially homes for bacterial communities. They are a sticky, thick matrix that aids in growth and allows for the uptake of nutrients while protecting the bacteria against threats (being washed away, antibiotics, etc.). To keep the bacteria alive, they have channels that allow nutrients and liquid to flow through.
Some examples:
- Plaque on teeth
- Slimy gunk that appears in a sink drain
- Can develop on medical implants, causing infection
Getting started
My research mentor and I decided that I would investigate the biofilms of B. avium and B. hinzii. A recently graduated student had been attempting to pursue this area, but they were having trouble getting their experiments to work and could not make any progress. Instead of picking up right where they left off, I ventured in a different direction, hoping to make some headway.
To start, I decided to go with a simple idea that would provide enough qualitative info to help guide our next decision: mix the two bacteria species in media and see what happens. I had two control solo cultures (B. avium and B. hinzii growing alone in tubes).
To our surprise, there was already a noticeable difference between the tubes within just a few days in the incubator. When grown together, the biofilm produced is thicker than that of solo cultures. This finding propelled my undergraduate research and narrowed my focus to the differences between these biofilms.
In the picture to the left, you can see my first experiment. The left tubes (white caps) were the “experimental” tubes with both B. avium and B. hinzii bacteria. The middle tubes have only B. hinzii, and the right tubes have only B. avium.
Looking around the tubes’ center, you’ll see a weird white substance. That’s the biofilm! (Technically, they’re “pellicles” because they float, but that’s mostly semantics)
The mixed tubes’ biofilms were so large that they began to collapse under their weight. When comparing this to the other two tubes, there’s a significant difference in the quantity of biofilm produced…
How do I quantify this difference?
Quantifying biofilms when the standard method won't work
The typical method for quantifying biofilms is called a Crystal Violet assay. For this, you grow biofilms and stain them with a liquid known as Crystal Violet, then use a machine to measure the amount of stain. The more stain, the more biofilm. Unfortunately, this assay did not work with these biofilms. I spent a couple of months troubleshooting ways to quantify these biofilms. A couple of things we tried included:
- Growing biofilms in tubes, drying them out, and weighing their mass
- Measuring the size and thickness of the biofilms
We eventually found a special fluorescent dye that binds to proteins and molecules in biofilms, which worked well with our bacteria.

To use this new fluorescent dye, I used 96-well-plates again. In each plate, I would have 4 different experimental samples:
- BH + BA mixed cultures
- BA solo cultures
- BH solo cultures
- Plain media with no bacteria
Overall, this worked well. There were significant differences in the fluorescence detected in these samples. However, there was one issue: data values between experiments were very variable. On some days, the values in a plate would read 5-10, but on other days, they would read 30-50. This was confusing. How do we compare inter-plate data when the numbers are so variable?
I went to the math department at JMU to get some advice, and I was recommended to convert all of my raw data points into Z-scores. Z-scores are a measurement of how far a data point is from the average of the data set. To calculate the individual Z-scores, I used the mean of the plate from which the data point came (as opposed to using the mean of all the data I got across all my experiments). This solved my problem because this essentially standardized my data.
Below are figures showing my results and hopefully making it easier to visualize. Some data points are different values, but that is just because the left figure (bar graphs) is more up-to-date and has more data. The right figure (bell curve) is a figure I made to explain more easily how z-scores work in relation to my research.
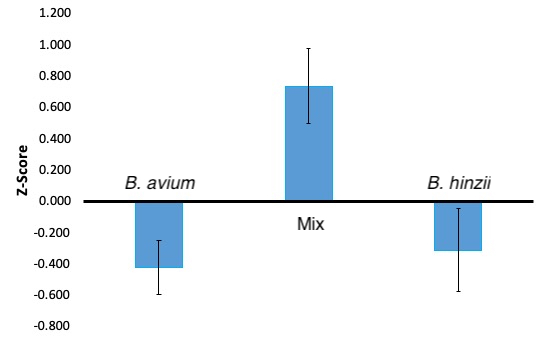
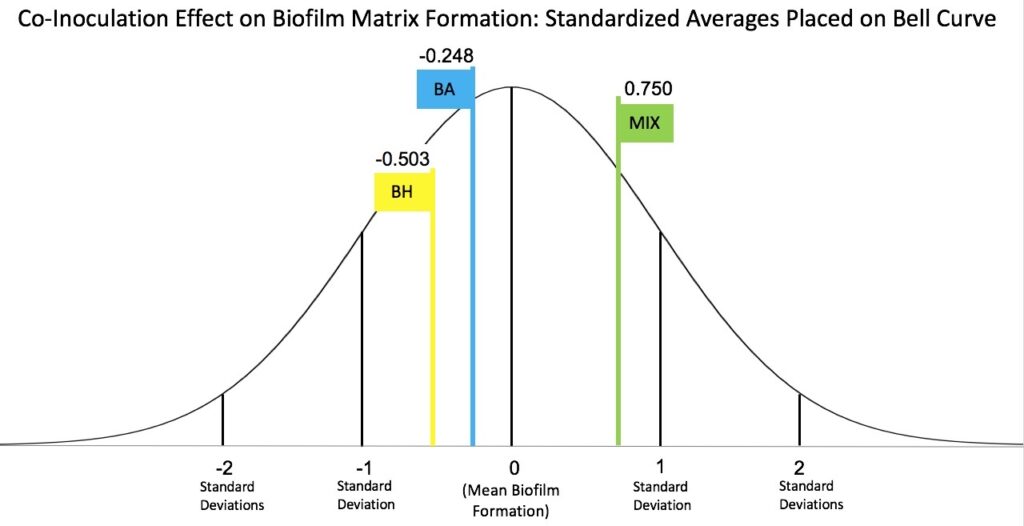
Key findings
Overall, I found that there is no significant difference in the amount of biofilm produced when comparing B. hinzii and B. avium solo cultures. However, when growing the two bacterial species together, there is a significant increase in biofilm produced.
How strong are these biofilms?
I reached a point in my research where I wanted to investigate the strength of these biofilms and answer questions like:
- Does B. hinzii produce a stronger biofilm than B. avium?
- Do B. avium and B. hinzii mixed cultures produce stronger biofilms than solo cultures?
This project was a bit of a challenge. When we dove into the literature, all means of testing biofilm strength required expensive and complicated equipment. We could not afford to invest so much time and money into a piece of equipment for just one project, so we had to design our own experiment.
We ended up creating what we called the “Drop Bead Test”. A very simple and cheap experiment, it will give you a preliminary idea of what’s going on. There are many uncontrollable or hard-to-control variables to keep in mind, so the room for error is larger than ideal. However, as long as you keep this in mind, it is a great way to get a general idea of how biofilms’ strengths compare. Another thing to remember: this tests tensile strength only (the resistance of material breaking under tension).
Protocol:
- Grow biofilms in tubes
- Drop 1-millimeter-sized beads onto the biofilms until the biofilms break
- Count how many beads they can hold
On the right, you can see a picture of the result of this experiment. In the middle of the tube, you can see the broken biofilms. You can see the glass beads at the bottom of the tubes after they fell through.
Key findings
Our results found that B. hinzii could hold 100+ beads, B. avium could hold 10-15 beads, and the mixed samples could hold 50-70.
I've got some questions
After conducting experiments on quantification and strength-testing, I was left with the following questions:
If B. avium and B. hinzii make roughly the same amount of matrix, why do they present so differently?
Could we see the differences between the biofilms? If so, could it help explain how or why they differ in strength?
Do they have different textures?
How does co-inoculation change the structure?
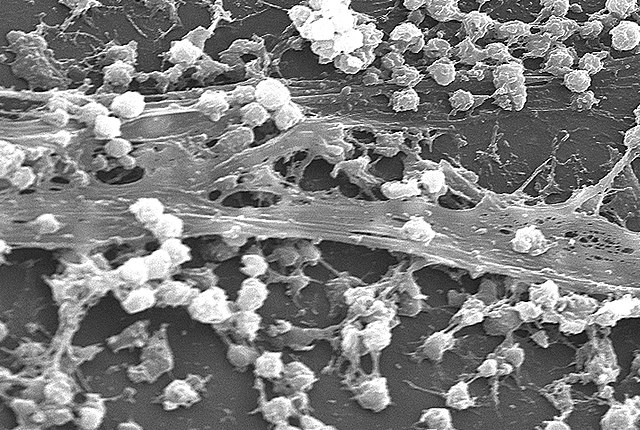
Microscopy: getting up close and personal
The next step in my research was to use fluorescence microscopy to try and get pictures of the biofilms. Fluorescence microscopy is when you stain a specimen with a fluorescent marker and then use a microscope that can see the fluorescence. Fortunately, I could use the same fluorescent stain that I used in the quantification assays for these experiments.
Finding a way to get the biofilms out of their growth media was challenging and required a lot of trial and error. I grew the biofilms in tubes (just like the strength testing assay) and then carefully “spilled” them out onto slides. Below is a picture of the result.

After figuring out how to get the biofilms onto the slides, it was time to bring them to the microscopy department. There, we used a Nikon TE2000 C2si Laser Scanning Confocal and Widefield Microscope. Below are some galleries displaying images we captured:
B. hinzii
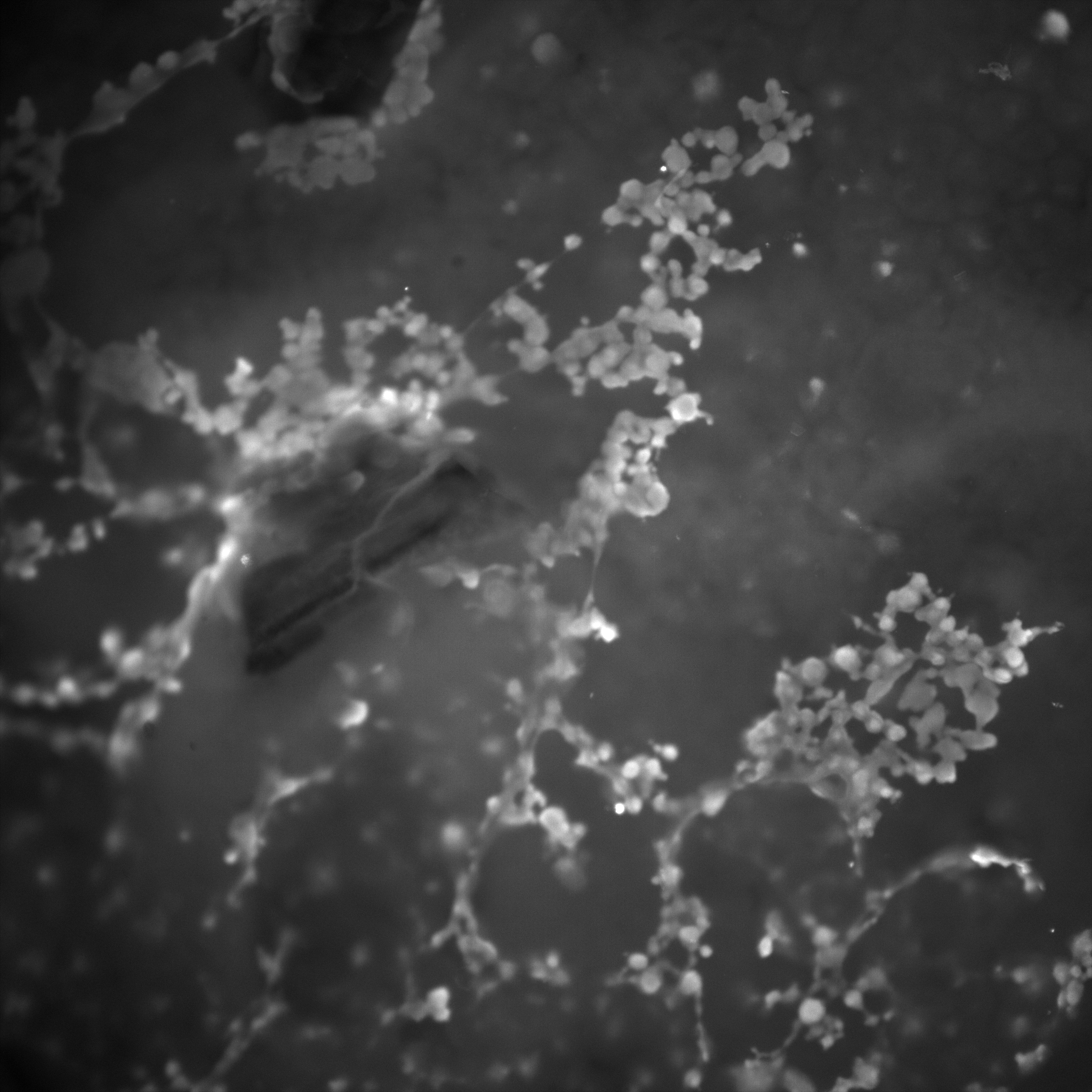
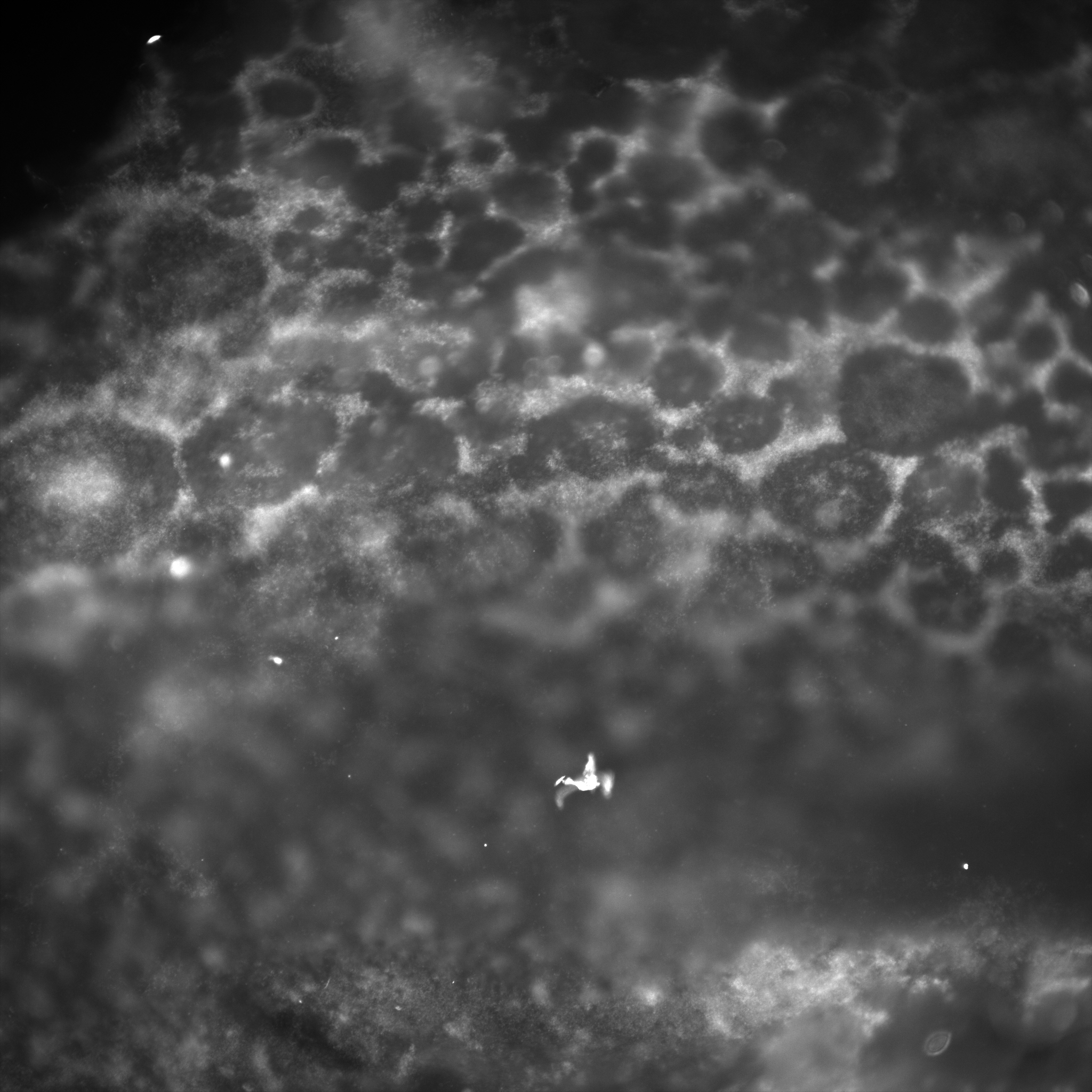
The following gallery includes pictures of B. hinzii biofilms. Notice the stringy, gummy-like texture in some areas, while others are fuzzier and lack defined edges. The numbers on the images’ overlays signify the magnification (_x).
B. avium
The following gallery includes pictures of B. avium biofilms. Notice that this texture is much more granular and defined than that of B. hinzii. Also, there is the presence of free-floating filamentous structures (white).
Mixed cultures
The following gallery includes pictures of biofilms from mixed cultures of B. avium and B. hinzii. Notice the frequent honey-comb-like structure and the presence of the same floating, filamentous structures.
To summarize what we saw looking at these pictures:
- There are significant qualitative differences in the biofilms of Bordetella avium and Bordetella hinzii
- When co-inoculated, the biofilm changes it structure in a way that varies from both B. avium and B. hinzii independent cultures
- Co-culture looks very organized
- In B. avium and mixed culture biofilms, there are distinctive spindly protein structures
- The function is unknown
Key findings
Microscopy shows significant differences in the structure and appearance of biofilms grown by B. hinzii, B. avium, and mixed cultures. B. hinzii is gummy, stringy, and undefined. B. avium is granular and thin. Mixed biofilms have a honeycomb-like structure. In both B. avium and mixed cultures, there are unknown filamentous structures floating around.
Where the research could go next
The microscopy experiments took place in my last year at JMU, but if I was going to continue working on this project, these are the questions I would try to answer:
- What is the spindly structure?
- Staining bacteria as well as biofilm matrix
- Where are the bacteria primarily located?
- Stain each species specifically – do they live closely or are they separated?
- What happens when you treat with antibiotics?
- Does co-culture affect antibiotic sensitivity?
- Test biofilm attachment to tracheal cilia
Overall, I really enjoyed these projects during my time at JMU and am so glad I got the opportunity to work in this lab.